DESIGN EXCELLENCE FOR MEDICAL DEVICES
Safety first
Combine simulations and preliminary tests to confidently select and test the most suitable design for production to reduce development time and cost of testing.
When it comes to the design and development of orthopedic and dental devices, nothing is more important than safety.
Engineers are tasked with creating products that practitioners and patients can rely on, so they must keep a tight grip on risk management throughout the product life cycle. They need to know how a prototype performs as quickly as possible in order to adapt and get to the best design quickly.
By shifting requirements and design data into a single digital platform, manufacturers can improve visibility at every level.
This means important decisions and outcomes from the risk analysis stage are written into the design and reflected in the end product, and it becomes easier to make changes during and after development.
With Siemens’ design excellence technology, you can combine simulations and preliminary tests to select and trial the most suitable design for production, reducing development time and the costs.
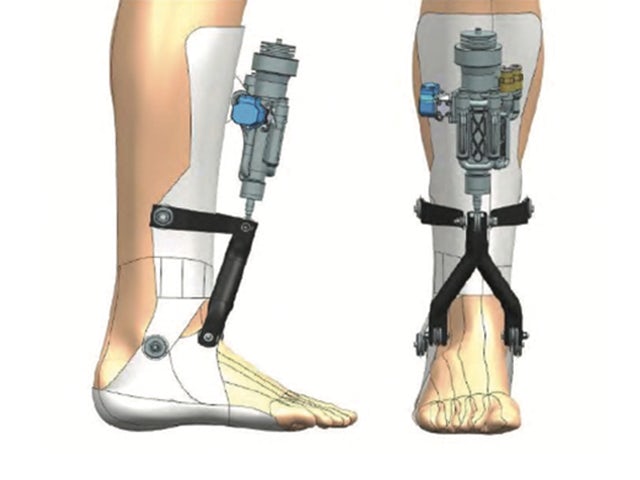
HES-SO University of Applied Sciences and Arts benefited from this approach when testing variants for its pioneering ankle exoskeleton. It was able to select the final design faster and reduce the cost of materials and resources in the testing phase.
Downloads
To discover what a renewed, holistic focus on design excellence could mean for your business, download our executive brief now.
Executive brief
The business benefits of collaborative design to navigate regulation. Siloed teams working on complex medical device design lead to delays. Learn how Siemens drives efficiency in the creation of safe, effective medical devices.
